Structure of JPMA
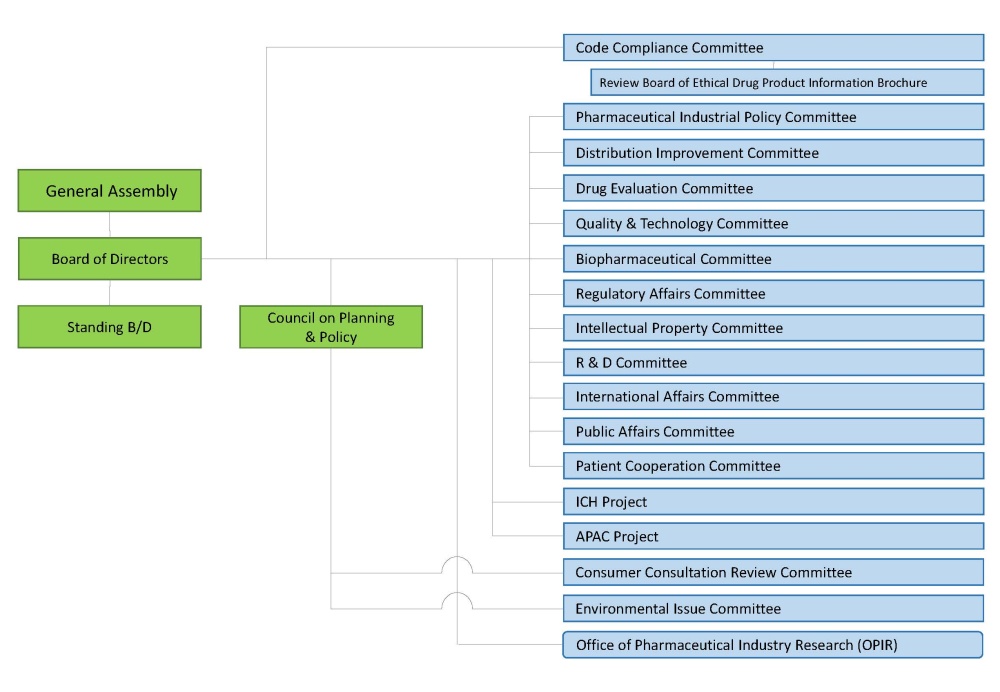
A click of a figure displays the expansion version.
Code Compliance Committee
Promotes the Compliance of JPMA Member Companies, maintains/manages the JPMA Code of Practice, and supports the JPMA Member Companies' activities on the compliance of code.
Review Board of Ethical Drug Product Information Brochure
To ensure the proper use of ethical drugs, we voluntarily review promotional materials such as summaries of ethical drug product information and specialized magazine advertisements prepared by member companies.
Pharmaceutical Industrial Policy Committee
To respond promptly to the changes in the environment surrounding the Pharmaceutical industry, conducts studies of industrial development, medical innovation, the tax program for research and development, and other issues relevant to industrial policy. Also examines any urgent matters to which other standing committees may not be able to respond.
Distribution Improvement Committee
Aims for accoutable and efficient distribution of drug products and related organizations and governmental bodies. Undertakes surveys and studies on drug product distribution for policy and strategy proposal.
Drug Evaluation Committee
Promotes appropriate measures for research and development of new drugs, post-marketing safety measures, appropriate use of drugs and medical affairs . Proposes policies and strategies based on consideration related to technology, regulatory science and regulation etc.
Quality & Technology Committee
We conduct surveys and studies on Good Manufacturing Practice (GMP), Pharmaceutical manufacturing and analytical technology, and related matters from an international perspective. We also establish and actively promote initiatives to improve the reliability of the quality of pharmaceutical products and to foster a Quality Culture. Through these activities, we contribute to the improvement of medical care and the sound development of the industry.
Biopharmaceutical Committee
Presents recommendations to governmental bodies on biopharmaceutical-related policies concerning the infrastructure development for promoting R&D of biotechnology-based drugs. Investigates technical issues in R&D, manufacturing, post-marketing surveillance, and recommends the relevant ministries/agencies on improvements.
Regulatory Affairs Committee
Investigates problems in implementing the Pharmaceutical and Medical Device Act, the approval process of drugs, and safety measures in pharmaceutical regulations, and recommends the pharmaceutical agencies on policies regarding to effective corporate activities and swift drug approval etc. from stand points of R&D-based pharmaceutical companies.
Intellectual Property Committee
Coodinates with relevant gorvernmental bodies and other organizations to promote an international competitiveness of Japanese Pharmaceutical industry. Proposes new policies and strategies to establish a virtual cycle of creation, protection, and utilization of intellectual property worldwide in Life Science.
R & D Committee
Aim to realize effective drug discovery R & D of member companies through gather and share information on priority issues in non-clinical and clinical trials from the research stage, propose measures to AMED and ministries, and promotion of open innovation.
International Affairs Committee
Based on our three guiding principles of (1) enabling international business development, (2) catalyzing international policy harmonization, and (3) strengthening contribution to global health, we will deepen collaboration with regulators, R&D-oriented industry associations in the target region and International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), and continue to implement activities for those challenges to address issues faced by industry and our member companies.
Public Affairs Committee
Develops activities to improve public recognition and understanding of medicines and the pharmaceutical industry. Takes action to facilitate adoption and implementation of JPMA proposals and policies.
Patient Cooperation Committee
Builds better relationships with patient groups and actively exchanges opinions with them, and disseminates information and executes policies that reflect the patients perspective. Furthermore, endeavors to make well-known the "JPMA Code of Practice on Relationships between the Pharmaceutical Industry and Patient Groups".
ICH Project
Joins “International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH)”, in which many regulatory authorities and global organization of pharmaceutical industries bring together to develop harmonized guidelines. The Project promotes better understanding of draft and finalized guidelines through regular capacity building activities, such as ICH Public Meeting, Implementation Training Workshops and ICH Forums that enable people to improve their skills and knowledge.
APAC Project
APAC = "Asia Partnership Conference of Pharmaceutical Associations" is a platform to share information considered to be problem among 13 R&D-based pharmaceutical member associations in Asia, then disseminate necessary advices/proposals for realizing the mission "To expedite the launch of innovative medicines for the peoples in Asia". At the annual APAC conference, expert working groups for "Regulations & Approvals", "e-labelling" and "Drug Discovery Alliances" as well as several task-force teams aim to generate consensuses toward achieving the mission by discussing the results of investigation, study, and work on themes set each, together with the regulators and academia.
Consumer Consultation Review Committee
Investigates and advises on appropriate ways to provide the public with drug information and on how product information departments should operate, to gain more reliability of the provide information and recognitions as a consultation, thus contributing to Patient-oriented Health Care.
Environmental Issue Committee
This committee aims to promote environmental protection activities by examining measures to promote responses to various issues related to global environmental protection.
Office of Pharmaceutical Industry Research (OPIR)
Conducts research on policies and various topics comprehensively for the development of the pharmaceutical industry and for its contributions to society from a medium to long term perspective.

